Abstract
Introduction: Systemic mastocytosis (SM) is a mast cell (MC) neoplasm driven by the KIT D816V mutation in approximately 95% of cases, resulting in MC hyperactivation and accumulation in various organs. Diagnosis of SM includes evaluation of MC aggregates in extracutaneous organs, atypical MC morphology and expression of CD25 with or without CD2, detection of the KIT D816V mutation, and a serum tryptase level of >20 ng/ml. In addition to these diagnostic parameters, expression of CD30 has been observed on neoplastic MCs in patients with SM. Avapritinib, a highly potent and selective KIT D816V inhibitor, is now approved by the FDA for use in advanced SM (AdvSM) based on the phase 1 EXPLORER and phase 2 PATHFINDER studies. In EXPLORER, avapritinib markedly reduced the bone marrow (BM) MC burden in patients with AdvSM. Here, we describe a comprehensive evaluation of the effect of avapritinib on MC burden, morphology, and immunohistochemistry in BM, BM cellularity and fibrosis, as well as changes in selected hematologic parameters in patients with AdvSM from the pivotal phase 2 open-label, single-arm PATHFINDER (NCT03580655) study.
Methods: Patients aged ≥18 years with centrally confirmed AdvSM were treated with avapritinib 200 mg once daily. Peripheral blood smears, bone marrow biopsies (BMBs), and aspirates (BMAs) were obtained at screening, and after 8 and 24 weeks. Evaluations of morphology were performed using standard Wright-Giemsa and H&E staining, while immunohistochemistry was performed on formalin-fixed EDTA-decalcified BM sections using standard techniques for tryptase, CD117, CD25, and CD30. Staining was also performed to detect reticulin and collagen fibrosis. Complete blood counts and differentials were performed at screening, and after 8 and 24 weeks.
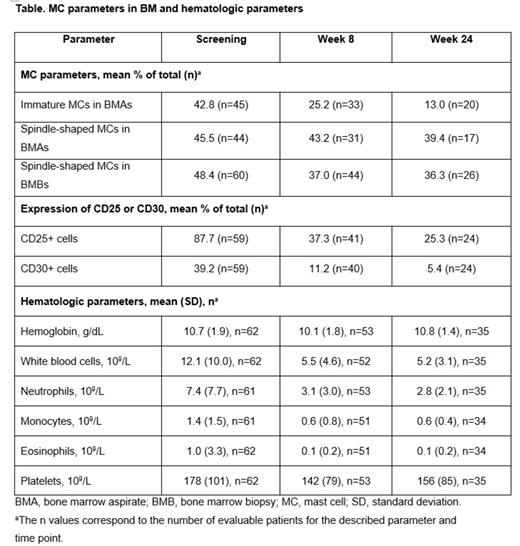
Results: In an interim analysis of PATHFINDER, the overall response rate (per modified International Working Group-Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis response) was 75% in 32 evaluable patients with AdvSM who received avapritinib. Reductions of ≥50% in BM MCs were observed in 88% (44/50) of patients and 60% of patients (30/50) had elimination of BM MC aggregates. Following treatment with avapritinib, an overall decrease in MC burden was observed in BMBs with a mean decrease of 64% at Week 8 (n=47) and 73% at Week 24 (n=24), and was associated with a decrease in the percentage of MC aggregates. Avapritinib treatment also resulted in reductions of the proportion of CD25+ and CD30+ MCs in BMBs by Week 8 and Week 24 (Table). In BMAs, avapritinib reduced the mean MC burden from 11% (out of total nucleated cells; n=47; range 0-72) at screening to 3% (range 0-47) by Week 8 (n=43) and 3% (range 0-30) at Week 24 (n=24), with decreases in immature and spindle-shaped MCs (Table). Of 5 patients with circulating MCs at screening and post-screening sample measurements (all with SM with associated hematologic neoplasm diagnoses), all had no detectable MCs by Week 8. Cellularity in BMBs decreased from a mean of 86% at screening to 68% at Week 8 and 59% by Week 24. Fibrosis was present in BMBs in 58 of 59 patients (98%) at screening, 40/44 (91%) at Week 8, and 22/25 (88%) at Week 24; reticulin fibrosis (MF score) and collagen fibrosis (Grade) was reduced during avapritinib treatment in those patients presenting with increased fibrosis. However, no significant changes in osteosclerosis scores were noted. In peripheral blood, a marked decrease in mean eosinophil counts and reductions in white blood cell, neutrophil, monocyte counts were also observed. Platelet counts decreased while hemoglobin levels remained relatively consistent through Week 24 (Table).
Conclusions: Avapritinib demonstrated rapid (Week 8) and profound (Week 24) reductions in neoplastic MC burden characterized by a reduction of the total MC burden in BMBs and BMAs with return to a normal morphologic appearance and normal immunophenotype, usually over 6 cycles. This was accompanied by a normalization of BM cellularity, as well as a decrease in fibrosis and marked improvement in hematologic parameters. These improvements in disease histopathology, along with known deep molecular remission of KIT D816V, suggest the potential for modification of AdvSM disease natural history.
George: Blueprint Medicines: Consultancy; Celgene: Consultancy; Bristol Meyers Squibb: Consultancy; Incyte Corporation: Consultancy. Fredericks: Blueprint Medicines Corporation: Current Employment. Reiter: Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; AOP Orphan Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Deciphera: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees. Radia: Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Study steering group member, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Education events; EXPLORER and PATHFINDER studies: Other: Member of the Response Adjudication Committee; Cogent Biosciences Incorporated: Other: Study Steering Committee. Deininger: Novartis: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fusion Pharma, Medscape, DisperSol: Consultancy; Incyte: Consultancy, Honoraria, Research Funding; SPARC, DisperSol, Leukemia & Lymphoma Society: Research Funding; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of a Study Management Committee, Research Funding. Lin: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Sen: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Gotlib: Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cogent Biosciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Chair for the Eligibility and Central Response Review Committee, Research Funding; Allakos: Consultancy; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dimitrijević: Blueprint Medicines Corporation: Current Employment, Current holder of individual stocks in a privately-held company. DeAngelo: Takeda: Consultancy; GlycoMimetics: Research Funding; Abbvie: Research Funding; Shire: Consultancy; Novartis: Consultancy, Research Funding; Forty-Seven: Consultancy; Incyte Corporation: Consultancy; Jazz: Consultancy; Pfizer: Consultancy; Blueprint Medicines Corporation: Consultancy; Autolus: Consultancy; Amgen: Consultancy; Agios: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal